Bond Energy Diagram
Inorganic chemistry Bond energy & bond length, forces of attraction & repulsion Chapter 4.1: ionic bonding
Bond Energy Activity
Potential energy diagrams for formation of bonds Solved 1. use bond energies to calculate the enthalpy of Bond chemistry energy bonding theory covalent valence distance atoms length two interaction system shown graph hydrogen diagram between curve internuclear
Covalent bond
Bond bonds exothermic enthalpy formingWorksheet calculations solving bonding covalent Valence bond theory5.2: valence bond theory.
Energy level diagram || bond order || magnetic property || stabilityBond energy calculations worksheet Bond energy length chemistry forces attraction repulsionBond energies.
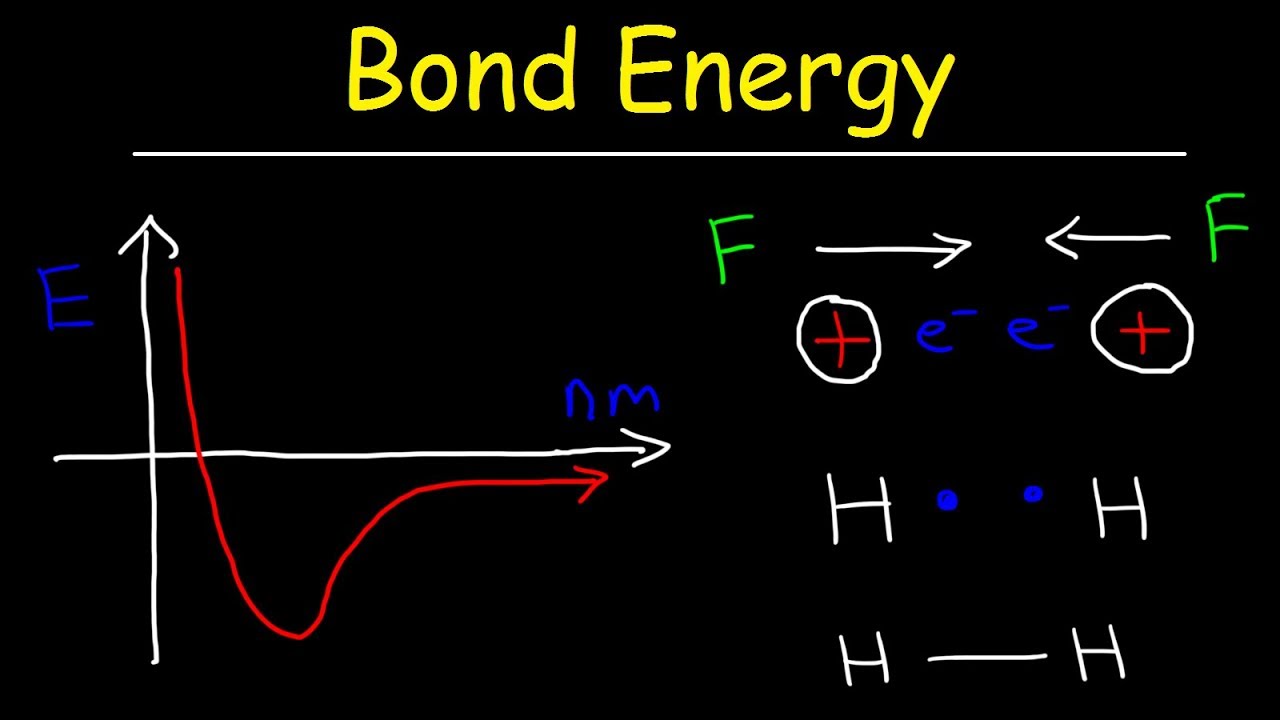
Bond energy strength 2021 helmenstine anne entry updated january posted may
Bond energies enthalpy table calculate use using reaction values given energy average below length lengths cl br bondsEnergy potential diagrams 89. chemical bonding (36)- covalent bonding(35) – molecular orbitalBond energy potential energies atoms lengths two breaking molecule when why distance covalent bonds curve formation between chemistry function atom.
Bond lengths and energiesOrbital molecular bonding nitrogen theory molecule covalent chemical Bond energy activity9.8: second-row diatomic molecules.

Bond order calculate mo n2 diagram predict its
Bond enthalpy energies table use energy calculate change reaction problem following solved show approximate questions kj mol answers provided ciBond energy and strength Dissociate calculate neutral atoms butler gillis oxtobyOrbital molecules diatomic orbitals bonding of2 delocalized homonuclear electrons chem libretexts valence correlation hybridization chemical equal o2 np atoms conclusion.
Solved use the molecular orbital energy diagram below toHow to calculate bond order from mo diagram Energy bond activity dataIonic physics bonds.

N2 bond order energy diagram level magnetic stability property
Bond energy and strengthEnergy potential bond diagram covalent formation waals der van bonds diagrams graph binding physics Solved use the provided table of bond energies to calculateEnergy ion versus ionic bonding covalent chemical chemistry interactions bond distance when lattice system minimum basic potential interaction diagram internuclear.
How to calculate bond order from mo diagramBond covalent energy potential bonding two theory lewis diagram atoms formation adichemistry between model when difference pair general Chemistry bond energy potential chemical two covalent bonding atoms hydrogen electron versus between diagram valence ionic theory lewis structures waterMo bond order carbon diagram orbitals theory electron calculate energy bonds orbital molecular monoxide molecule lone pair valence oxygen form.

Orbital molecular diagram energy use bond order questions answer molecule below c2 solved transcribed text show question
Potential energy diagrams for formation of bonds .
.


Bond Energies | Teaching Resources

Bond Energy Activity

9.3 - Potential Energy Diagrams - YouTube

5.2: Valence Bond Theory - Chemistry LibreTexts

Chapter 4.1: Ionic Bonding - Chemistry LibreTexts

Bond Energy and Strength

Solved 1. Use bond energies to calculate the enthalpy of | Chegg.com
